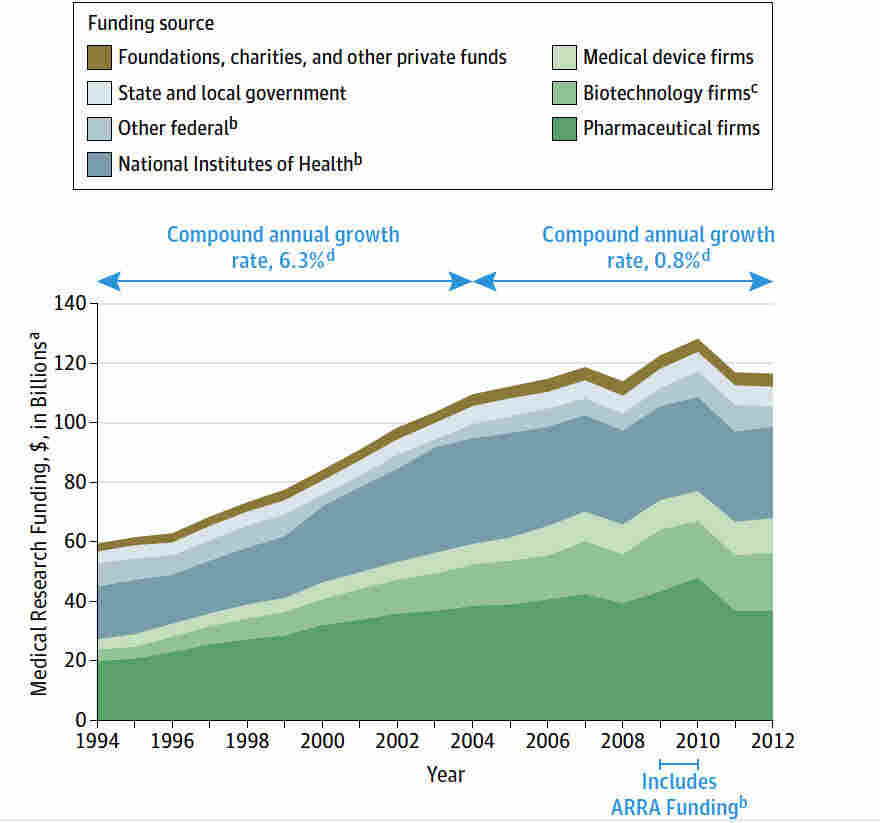
Medical research funding is critical for the ongoing safety and ethical oversight of clinical studies involving human participants. Recent cuts to federal research grants, particularly over $2 billion frozen by the Trump administration, have severely disrupted vital work conducted by institutions like Harvard. Such funding is essential not only for facilitating studies but also for ensuring compliance with medical ethics oversight and participant safety protocols. The impact of funding cuts on research extends beyond the immediate loss of resources, affecting the integrity of institutional review boards (IRBs) that play a crucial role in safeguarding research participants. As these financial constraints threaten to halt progress in medical innovation, the entire healthcare community must grapple with the far-reaching consequences on patient safety and trust.
The landscape of medical research financing plays a pivotal role in the pursuit of healthcare advancements and patient protection. With significant federal grants being curtailed, institutions face obstacles in sustaining their research initiatives, which complicates ethical scrutiny and participant monitoring. Funding limitations not only challenge the operational capabilities of research teams but also hinder the essential functions of oversight committees, influencing how clinical studies are designed and conducted. As a consequence, the ripple effects of diminished funding could undermine public confidence in research integrity and the safety of clinical trials. This ongoing situation highlights the interconnection between financial support, ethical oversight, and the health outcomes of participants involved in scientific studies.
Understanding the Role of Medical Research Funding
Medical research funding is crucial for advancing scientific knowledge and developing new therapies that can improve patient care. Federal grants, particularly from the National Institutes of Health (NIH), constitute a substantial portion of funding for research institutions like Harvard, which relies on these funds to support research infrastructure and oversight. The ability to conduct high-quality clinical trials depends on adequate financial resources, which enable institutions to maintain rigorous oversight, ensure participant safety, and adhere to ethical standards throughout the research process.
The recent halt in federal research grants not only impacts the financial stability of research institutions but also threatens the integrity of ongoing studies. Funding cuts can lead to delays in research protocols, disrupt patient recruitment efforts, and limit the ability of institutional review boards (IRBs) to function effectively. Without financial assistance, these boards may struggle to fulfill their critical roles in protecting participants’ rights and safety. Thus, ensuring steady funding for medical research is essential for fostering innovation and safeguarding public health.
Impact of Funding Cuts on Clinical Research and Patient Safety
The ramifications of funding cuts extend beyond administrative hurdles; they pose real risks to participant safety in clinical trials. Disruptions in funding can lead to interruptions in necessary oversight from IRBs, which are responsible for safeguarding the welfare of patients enrolled in studies. With reduced resources, IRBs may not be able to adequately review and monitor studies, increasing the potential for ethical breaches and compromising participant safety. Historical precedents demonstrate how lapses in oversight can lead to serious ethical violations, making it imperative that IRBs maintain robust levels of support.
Furthermore, the cancellation of research grants directly affects the trust of participants. When individuals decide to engage in clinical trials, they do so with the expectation of protection and ethical consideration from the research institutions involved. Cuts in funding can erode this trust, prompting skepticism about the research landscape and its commitment to ethical standards. This could deter potential volunteers from participating in crucial studies, ultimately impeding progress in medical advancements and research outcomes.
The Role of Institutional Review Boards (IRBs) in Research Oversight
Institutional Review Boards (IRBs) play an indispensable role in upholding the ethical standards of medical research. By providing thorough evaluations of research proposals, IRBs ensure that studies prioritize the safety and rights of participants above all else. The IRB’s responsibilities include assessing potential risks, ensuring informed consent, and monitoring ongoing research for compliance with ethical guidelines. In this capacity, they act as a vital checks-and-balances system that upholds the integrity of the research process and protects individuals who volunteer for studies.
In light of recent federal funding cuts, the functionality of IRBs is increasingly threatened. The loss of resources can impede their ability to conduct timely reviews and maintain standards of oversight necessary for patient safety. A well-funded IRB can efficiently manage the complexities of modern clinical trials, including sIRB reviews for multisite studies, which are essential in today’s collaborative research environment. Therefore, financial stability for IRBs is not merely a bureaucratic concern; it significantly influences the protection of participants and the overall ethical landscape of clinical research.
Federal Grant Cuts and Their Long-Term Consequences
Federal research grant cuts create a ripple effect that can harm not just current research projects but also the broader landscape of medical innovation. When funding is halted, numerous studies across various institutions face delays or are forced to shut down entirely. This loss impacts the development of critical treatments and therapies, stalling progress that could save lives and improve health outcomes. Researchers may find themselves unable to secure necessary resources, resulting in a slower pace of discovery and a diminished pipeline of new medical advancements.
Moreover, the ramifications of these funding cuts can extend beyond immediate financial implications. As ongoing research projects face interruptions, public trust in the research enterprise may be eroded. Participants who are willing to volunteer for trials often do so due to a belief in the integrity and ethical standards of the institutions conducting the research. If funding cuts lead to compromised oversight and eroded standards, communities may become skeptical of clinical trials, ultimately discouraging participation and slowing the pace of scientific innovation.
Preserving Patient Rights Amidst Research Funding Challenges
The preservation of patient rights and welfare is paramount in clinical research, especially amid increasing funding challenges. In the face of budget constraints, research institutions must prioritize the ethical considerations and protections afforded to participants. Institutional Review Boards (IRBs), in particular, are tasked with ensuring that all human subjects research complies with ethical standards aimed at safeguarding participants’ rights, health, and overall well-being. Proper oversight facilitated by adequate funding is essential to provide the necessary support for these vital functions.
Failure to secure sufficient resources for IRBs could lead to lapses in the protective measures safeguarding research participants. This places individuals at heightened risk for potential harm or ethical breaches, which can compromise the validity of the research outcomes. Moving forward, it is critical for research institutions and policymakers to advocate for stable funding sources, ensuring that patient rights remain at the forefront of research ethics and that the trust between researchers and participants is maintained.
The Importance of Ethical Oversight in Medical Research
Ethical oversight is a fundamental tenet of medical research that ensures the protection of participants involved in clinical trials. Through rigorous review processes by IRBs, researchers are held accountable to standards that prioritize participant safety and informed consent. In a landscape increasingly affected by funding cuts, the need for strong ethical oversight has never been more pressing. Effective oversight mechanisms are crucial in preventing past abuses from resurfacing and maintaining public confidence in the research enterprise.
In light of historical abuses in medical research, including infamous cases like the Tuskegee Syphilis Study, the establishment of ethical standards and oversight has been essential. These systems of governance work to create frameworks that keep individuals informed and protect their rights to participate voluntarily in research. Continual funding support for ethical oversight mechanisms is necessary to ensure that such frameworks remain operationally robust, allowing the research community to uphold the highest standards and maintain the trust of the public.
Revitalizing Public Trust in Medical Research
Public trust is critical for the success of clinical trials and the broader acceptance of medical advancements. Funding cuts can jeopardize the relationship between research institutions and the public, as delays and ethical lapses may lead to skepticism regarding the integrity of research practices. Establishing transparent communication about how funds are allocated and ensuring participant safety should be priorities for researchers. By demonstrating commitment to ethical practices and effective oversight, institutions can work towards rebuilding trust among potential research participants.
Additionally, rebuilding public trust might involve engaging communities in research discussions and emphasizing the positive impacts of well-conducted studies. Transparent reporting of outcomes and ethical inquiries is essential to convey the message that participant involvement is valued and respected. Ensuring that community voices are heard and involved in research can help mitigate mistrust and foster collaborative relationships between researchers and participants. Without public trust, the potential for groundbreaking medical discoveries could remain untapped, underscoring the need for renewed focus on ethical standards.
Addressing the Future of Medical Research Funding
As funding cuts continue to present formidable challenges to medical research, a collective effort is needed to address the future of research financing. Advocacy for increased funding from federal agencies, alongside the exploration of alternative financial models, is crucial for sustaining research initiatives. Collaborative partnerships among institutions, private stakeholders, and non-profit organizations can help create innovative funding solutions that prioritize the advancement of medical research while ensuring the protection of study participants.
In the face of evolving challenges, it is natural for the medical research community to seek out new avenues for funding that align with ethical standards and accountability. By fostering a culture of transparency and rigorous evaluation, research institutions can promote confidence in their funding arrangements. Ultimately, the aim should be to create a resilient funding ecosystem that supports innovative and safe medical research, thereby enhancing the future of healthcare advancements and the well-being of patients.
Concluding Thoughts on the Need for Reliable Research Funding
In conclusion, the health of the medical research landscape relies heavily on the availability of reliable funding. The implications of research funding cuts are widespread, jeopardizing not only ongoing studies but also the broader framework of patient safety and ethical oversight. It is imperative for policymakers and stakeholders to recognize the critical need for sustained funding, ensuring that research institutions can continue to uphold ethical standards and protect the rights of study participants.
Without a firm commitment to supporting medical research through consistent and reliable funding, the consequences can be dire, affecting not only the progress of scientific inquiry but also the safety and trust of individuals who participate in research. Moving forward, it is essential to prioritize conversations around funding, ethics, and oversight to safeguard the future of medical advancements and ultimately enhance the health and safety of communities across the nation.
Frequently Asked Questions
What is the impact of funding cuts on medical research efforts?
Funding cuts significantly hinder medical research by disrupting essential oversight systems like institutional review boards (IRBs). When federal research grants are reduced, the ability to ensure participant safety, monitor ethical compliance, and conduct critical studies on various diseases suffers greatly. This can lead to halted projects, undermined public trust, and ultimately delayed medical advancements.
How do federal research grant cuts affect clinical research participant safety?
Federal research grant cuts jeopardize clinical research participant safety by limiting the resources available for oversight by IRBs. A reduction in funding can lead to mid-study halts, increased risk of ethical breaches, and a lack of necessary safety protocols, diminishing the quality of care and protection for participants engaged in medical studies.
What role does an IRB play in medical research funding?
An Institutional Review Board (IRB) plays a critical role in medical research funding by ensuring that all studies comply with ethical standards and regulations. When research funding is adequate, IRBs can effectively monitor studies, protect participants’ rights, and mitigate risks. However, funding cuts can undermine IRB functions, potentially leading to increased risks for research participants.
What are the implications of the impact of funding cuts on research ethics?
The impact of funding cuts on research ethics can be profound. Reduced funding may force research institutions to compromise on oversight practices mandated by IRBs, leading to potential ethical breaches. Ensuring compliance with medical ethics oversight becomes challenging when financial resources are scarce, which can harm participants and undermine public confidence in medical research.
How does medical research funding compliance with IRB influence patient safety?
Medical research funding compliance with IRB standards is crucial for patient safety. IRBs assess research proposals to evaluate risks and benefits, ensuring informed consent and participant protection. When funding cuts impair IRB operations, the quality of oversight declines, increasing the risk of patient harm and unethical practices in medical research.
In what ways can the halt in funding impact the advancement of medical research?
A halt in funding can significantly curtail the advancement of medical research by disrupting ongoing studies and reducing the capacity of IRBs to provide oversight. This can lead to delays in critical research projects, neglect of participant safety protocols, and the stifling of innovative therapeutic developments that depend on collaborative efforts across research institutions.
What are potential consequences of federal research grant cuts on community trust in medical research?
Federal research grant cuts can erode community trust in medical research by causing mid-study interruptions and fostering skepticism about research practices. As studies are canceled or delayed, public confidence in the integrity and safety of clinical trials diminishes, which can discourage participation and skew the valuable data necessary for medical advancements.
| Key Point | Description |
|---|---|
| Funding Freeze Impact | The Trump administration froze over $2 billion in federal research grants, disrupting efforts to protect patient rights and safety. |
| Role of IRBs | Institutional Review Boards (IRBs) are responsible for overseeing the protection of research participants, ensuring compliance with ethical standards. |
| Historical Context | Past unethical medical research prompted the establishment of IRBs to safeguard participant rights and safety. |
| Consequences of Cuts | Cuts to funding can lead to halted studies, increased risks to participants, and a decline in public trust in medical research. |
| Ongoing Support | Despite funding cuts, Harvard Medical School is continuing to support collaborative research to mitigate negative impacts. |
Summary
Medical research funding is essential for protecting the rights and safety of patients involved in clinical studies. The recent freeze on federal grants has greatly undermined the capacity of research institutions to carry out necessary oversight, thus jeopardizing patient welfare. With funding cuts threatening to halt critical research, it is imperative that stakeholders work collaboratively to restore support for medical research funding to ensure continued advancements in healthcare and patient protection.