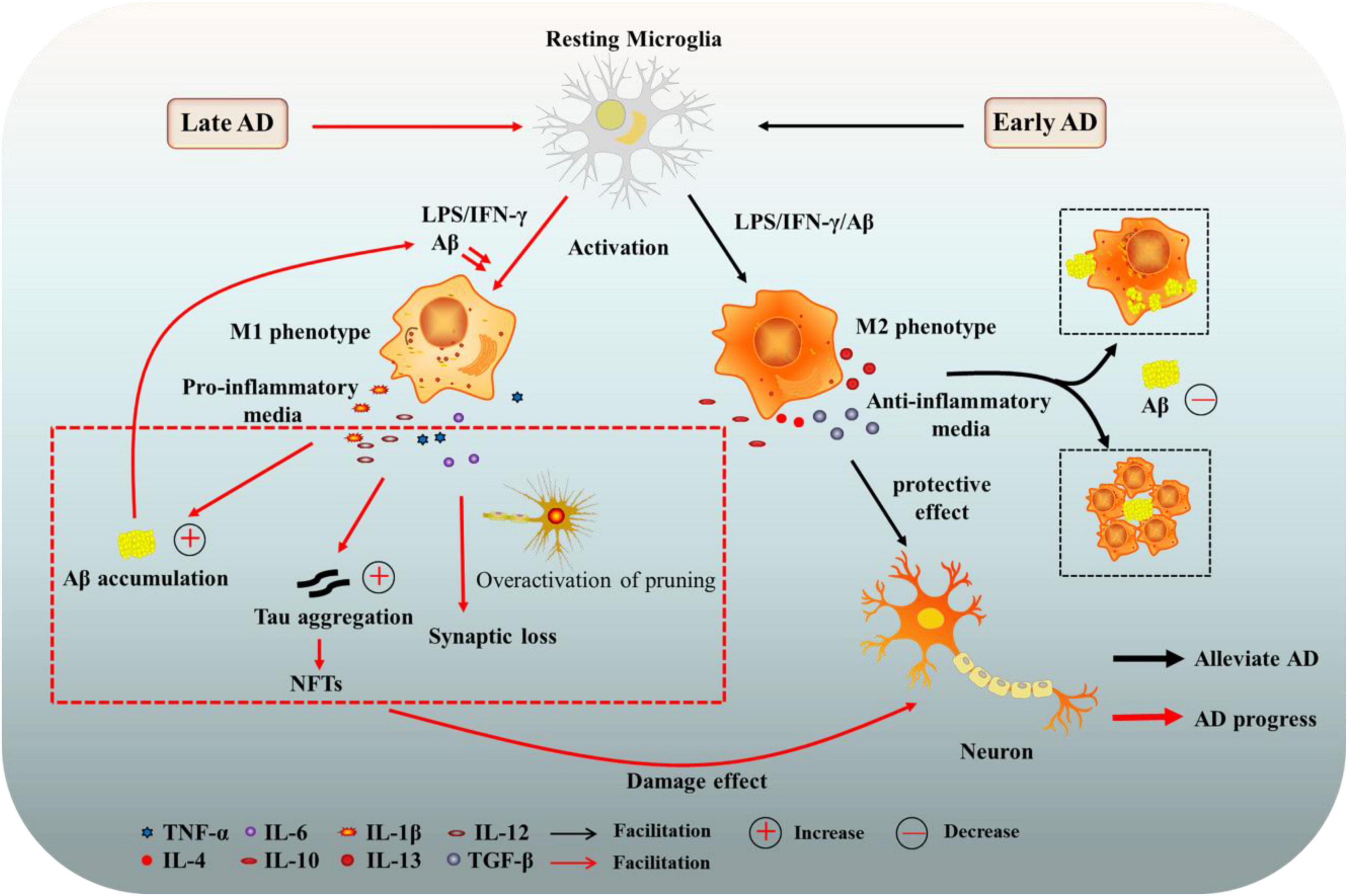
Microglia and Alzheimer’s disease are at the forefront of groundbreaking research aimed at unraveling the complexities of neurodegenerative diseases. As the brain’s primary immune cells, microglia play a crucial role in maintaining neuronal health by facilitating synaptic pruning and monitoring for cellular damage. However, recent studies suggest that dysregulation in microglial function can exacerbate conditions like Alzheimer’s, leading to further cognitive decline. Research spearheaded by Beth Stevens at the Broad Institute highlights the potential of microglial signaling pathways as targets for innovative neurodegenerative disease treatments. With over 7 million Americans afflicted by Alzheimer’s, understanding the intricate relationship between microglia and Alzheimer’s is essential for advancing Alzheimer’s research and developing effective interventions.
The role of brain immune cells, particularly those known as microglia, has become a pivotal focus in understanding Alzheimer’s and other age-related dementias. These immune system guardians not only clean up cellular debris but also dynamically sculpt neuronal networks through a process called synaptic pruning. However, abnormalities in microglial activity can trigger neuroinflammation, potentially worsening the symptoms of Alzheimer’s disease. Researchers like Beth Stevens have been instrumental in identifying these mechanisms, laying the groundwork for future innovations in treatment strategies. By exploring the intricate interactions within the brain’s immune architecture, scientists aim to uncover new pathways that could lead to breakthroughs in managing Alzheimer’s and similar disorders.
The Role of Microglia in Alzheimer’s Disease
Microglia act as the brain’s immune system, constantly on the lookout for signs of neurodegeneration such as those seen in Alzheimer’s disease. These specialized cells facilitate the maintenance of brain health through a process known as synaptic pruning. By eliminating weak or unnecessary synapses, microglia support the efficiency of neural communications. However, in the context of Alzheimer’s, this process can go awry, resulting in excessive synaptic pruning that may contribute to cognitive decline and memory loss.
Research led by Beth Stevens has uncovered the complexities of microglia behavior, illustrating how their actions can shift from protective to destructive in neurodegenerative diseases. By understanding the mechanisms behind microglial dysfunction, scientists hope to develop targeted therapies that can restore normal synaptic pruning, potentially reversing or mitigating the effects of Alzheimer’s disease. This research opens new pathways for neurodegenerative disease treatment, emphasizing the critical role these cells play in maintaining brain homeostasis.
Neurodegenerative Disease Treatment Innovations
The landscape of neurodegenerative disease treatment is rapidly evolving, particularly with advancements in Alzheimer’s research. Traditional approaches have focused on symptom management; however, recent studies suggest that targeting the underlying pathological processes could yield more effective therapies. Investigations into microglia function and their role in neurodegeneration are at the forefront, providing insights that could lead to innovative treatment strategies that directly address the root causes of diseases like Alzheimer’s.
By leveraging discoveries related to microglial activity, scientists are exploring new modalities of care that aim to enhance brain resilience. Research into biomarkers linked to microglial dysfunction is particularly promising, as these could aid in early diagnosis and treatment. As the scientific community continues to unravel the intricate relationship between microglia and neurodegenerative conditions, we inch closer to breakthroughs that may alter the course of diseases like Alzheimer’s, offering hope to millions affected around the globe.
Beth Stevens and her team at the Stevens Lab exemplify this innovative spirit, utilizing funding from the National Institutes of Health to propel their research forward. By pursuing curiosity-driven science, they have laid the groundwork for future therapies that not only seek to alleviate the symptoms of neurodegenerative diseases but also to understand and rectify the cellular malfunctions that drive them.
Understanding the Brain’s Immune System
The brain’s immune system, composed primarily of microglia, plays a fundamental role in how our neural architecture is formed and maintained. Microglia are not just passive observers; they actively participate in synaptic pruning, ensuring that only the most effective neural connections are preserved. This critical function supports cognitive processes and overall brain health, particularly during periods of learning and adaptation.
However, when microglial activity becomes dysregulated, it can lead to detrimental consequences for brain function. In diseases such as Alzheimer’s, altered microglial responses and misfiring synaptic pruning processes can accelerate neurodegeneration. Understanding how to modulate these cells and their impact on neural circuitry represents a promising frontier in research, with the potential to unlock new therapeutic avenues for managing conditions where the brain’s immune system fails.
Synaptic Pruning and Neural Health
Synaptic pruning is a vital process during brain development and plasticity, where microglia help refine and optimize neural connections. This biological mechanism ensures that the most efficient pathways are established, promoting effective communication between neurons. Such pruning is crucial for learning and memory, and any disruption in this process can have profound effects on cognitive function.
In the context of Alzheimer’s disease, improper synaptic pruning conducted by hyperactive microglia can lead to the loss of critical neural connections, exacerbating symptoms and accelerating cognitive decline. Research is now focusing on how to manipulate this process, with the hope that pharmacological interventions targeting microglial activity can restore balance in synaptic dynamics and enhance cognitive resilience in individuals at risk for or suffering from Alzheimer’s.
Beth Stevens’ Impact on Alzheimer’s Research
Beth Stevens’ pioneering work in the field of Alzheimer’s research has fundamentally shifted how the scientific community views microglia and their function within the brain. By investigating the critical role these cells play in synaptic pruning, she has opened up new avenues of exploration that bridge basic science with disease pathology. Her findings not only contribute to the understanding of Alzheimer’s but also impact broader neurodegenerative disease treatment approaches.
Through her research at the Stevens Lab, Stevens has showcased how insights gained from studying microglial behavior can inform the development of therapeutic interventions aimed at restoring healthy brain function. Her journey illustrates the importance of federal support, which has enabled vital discoveries that could translate into meaningful treatments for millions suffering from neurodegenerative diseases, including Alzheimer’s.
Biomarkers for Neurodegenerative Diseases
The search for reliable biomarkers in Alzheimer’s research is a critical aspect of advancing neurodegenerative disease treatment. Biomarkers facilitate early diagnosis and help track disease progression, making them invaluable for therapeutic development. Stevens’ work highlights the relationship between microglial dysfunction and the manifestation of neurodegenerative diseases, paving the way for novel biomarkers that reflect microglial health and synaptic integrity.
By focusing on the mechanisms of synaptic pruning and microglial activity, researchers are hopeful that they can identify specific biological markers associated with Alzheimer’s and other neurodegenerative diseases. These findings could greatly enhance our ability to diagnose these conditions earlier, tailor interventions more effectively, and ultimately improve patient outcomes, offering a glimpse of hope for those affected by these challenging disorders.
The Future of Alzheimer’s Research
Alzheimer’s research is at a pivotal crossroads, with advancements in our understanding of the brain immune system and microglial function leading the charge. As we uncover more about how microglia contribute to neurodegenerative processes, there is growing optimism that targeted interventions may soon emerge from the laboratory and into clinical practice. The potential for developing new therapies that not only address symptoms but also alter disease trajectories is at hand.
Researchers like Beth Stevens are crucial to this evolution, advocating for continued investment in basic science to drive innovation in Alzheimer’s treatment. As we look to the future, their work emphasizes the need to merge curiosity-driven research with translational efforts to ensure that discoveries made in the lab translate effectively into treatment opportunities that can benefit the millions affected by Alzheimer’s and other neurodegenerative diseases.
Funding Innovations in Alzheimer’s Research
The role of federal funding, particularly from the National Institutes of Health, cannot be understated in the realm of Alzheimer’s research. Financial support not only empowers scientists like Beth Stevens to pursue groundbreaking studies but also fosters an environment where innovative ideas can flourish. The foundation laid by these investments has been critical to producing discoveries that are shaping the future of neurodegenerative disease treatment.
Investment in Alzheimer’s research encourages interdisciplinary collaboration, allowing neuroscientists, geneticists, and clinicians to unite in the fight against this devastating disease. Understanding the relationship between microglia and neurodegenerative conditions is just one piece of the puzzle that federal funding is helping to unlock, providing hope for new therapeutic avenues that can ultimately improve the lives of those impacted by Alzheimer’s and similar disorders.
The Importance of Basic Science in Alzheimer’s Research
Basic science serves as the bedrock of all significant advancements in medicine and treatment. In Alzheimer’s research, foundational studies exploring microglial behavior and its effects on synaptic health are essential for understanding the disease’s complexities. Researchers like Beth Stevens highlight the need for a thorough investigation into the mechanisms of microglial actions, which can lead to pivotal insights and breakthroughs in neurodegenerative disease treatment.
The process of following scientific curiosity often leads to unexpected discoveries that can revolutionize our approaches to treating diseases like Alzheimer’s. These fundamental inquiries not only enrich our knowledge but also assist in guiding future research directions, ultimately improving patient care and outcomes. Basic science, therefore, remains a crucial element that empowers researchers to uncover transformative treatments for neurodegenerative diseases.
Frequently Asked Questions
What is the role of microglia in Alzheimer’s disease?
Microglia serve as the brain’s immune system and play a crucial role in Alzheimer’s disease by monitoring neuronal health. They help clear damaged cells and prune synapses, which are essential for efficient communication between neurons. However, in Alzheimer’s, aberrant microglial activity can contribute to disease progression by excessively pruning healthy synapses, leading to cognitive decline.
How does synaptic pruning by microglia relate to Alzheimer’s research?
Synaptic pruning by microglia is a normal process during brain development, but in Alzheimer’s research, it has been identified that dysregulated pruning may lead to the loss of functional synapses. Studies, including those led by Beth Stevens, propose that understanding this process can unveil new therapeutic targets and biomarkers for detecting and treating neurodegenerative diseases like Alzheimer’s.
What implications does Beth Stevens’ microglial research have for neurodegenerative disease treatment?
Beth Stevens’ research on microglia has significant implications for neurodegenerative disease treatment, particularly Alzheimer’s. By uncovering how microglia interact with synapses and their role in disease pathology, her work lays the groundwork for developing novel therapeutic strategies aimed at restoring normal microglial function, potentially reversing or mitigating Alzheimer’s pathology.
Can aberrant microglial activity serve as a biomarker for Alzheimer’s?
Yes, aberrant microglial activity can serve as a potential biomarker for Alzheimer’s disease. The research from Beth Stevens’ lab suggests that altered synaptic pruning and microglial function may provide critical insights into the early stages of Alzheimer’s, enabling earlier diagnosis and the development of targeted treatments that address the underlying immune responses in the brain.
What research is being done to understand the connection between microglia and Alzheimer’s disease?
Current research, particularly that of Beth Stevens, focuses on the mechanisms by which microglia contribute to Alzheimer’s disease pathology. Studies examine how microglial activation and synaptic pruning can lead to neurodegeneration and cognitive deficits, aiming to identify new therapeutic opportunities that can help manage or reverse the effects of Alzheimer’s.
| Key Points | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, monitoring for illness or injury and cleaning up damaged cells. |
| Aberrant Pruning | Improper synaptic pruning by microglia can contribute to neurodegenerative diseases including Alzheimer’s. |
| Impact of Research | Research by Beth Stevens has led to new biomarkers and potential medicines for treating Alzheimer’s. |
| Funding and Support | Federal funding, particularly from the NIH, has been crucial in supporting foundational research. |
| Future Potential | Further research on microglia may lead to advanced treatments that can improve the lives of those living with Alzheimer’s. |
Summary
Microglia and Alzheimer’s are intricately linked, as recent research illustrates the vital role of microglial cells in brain health and disease. Understanding these immune cells is paramount for developing new strategies to combat Alzheimer’s disease, which currently affects millions. Through pioneering work, scientists like Beth Stevens are uncovering how the malfunctioning of microglia can lead to neurodegeneration. As we learn more about their function and mechanism, we move closer to finding effective treatments for individuals affected by Alzheimer’s.